 Corn Field by Rastoney / BY NC
Corn Field by Rastoney / BY NCAt its core, my research agenda centers on the question of how governments regulate science, technology, and public health. Among my main interests is the regulation of new scientific advancements, especially developments in the field of biotechnology. A significant part of my work over the past two years has emphasized the debates over the effects of genetically modified organisms (GMOs) on public health and whether GMOs provide a pathway to environmental sustainability or to environmental degradation – central themes of my dissertation. My most recent presentation in Utrecht, on the evolution of the precautionary principle in EU food safety regulation, thus stays in line with my doctoral research. In addition to expanding on key themes within it, my dissertation has provided me with an excellent foundation for building a long-term research agenda on biotechnology regulation more broadly. As a result, I have also developed new research that examines biotechnology risk regulation in the field of reproductive medicine. Current projects include studies on genetic testing procedures, namely preimplantation genetic diagnosis (PGD), used during in vitro fertilization (IVF). The main focus of this work is the piecemeal regulatory approach in United States, and the ethical and legal challenges that arise from the lack of clear and comprehensive federal regulations. From this branch of my research agenda, I have developed several writing projects, including an article on the potential impacts of the implementation of the Affordable Care Act on reproductive health delivery (forthcoming in Politics and the Life Sciences) and a chapter in the collection I co-edited, Biopolitics and Utopia: An Interdisciplinary Reader.
To understand the relationship between the different branches of my research agenda, it is important to review its foundation: Biotechnology Regulation in the European Union and France: Un Dialogue des Sourds. My dissertation examined the case of French resistance to GMOs and the repercussions of France’s entrenched non-compliance with EU legislation and regulation in this policy domain. For this project, I pursued research on policy implementation processes and the role of supranational, national, and sub-national institutions in the context of EU regulation using an historical institutionalist approach combined with policy analysis. In order to understand this issue, my dissertation explored the multi-sectoral nature of agricultural biotechnology regulation and engaged with questions of environmental sustainability, risk regulation, public health, and food safety.
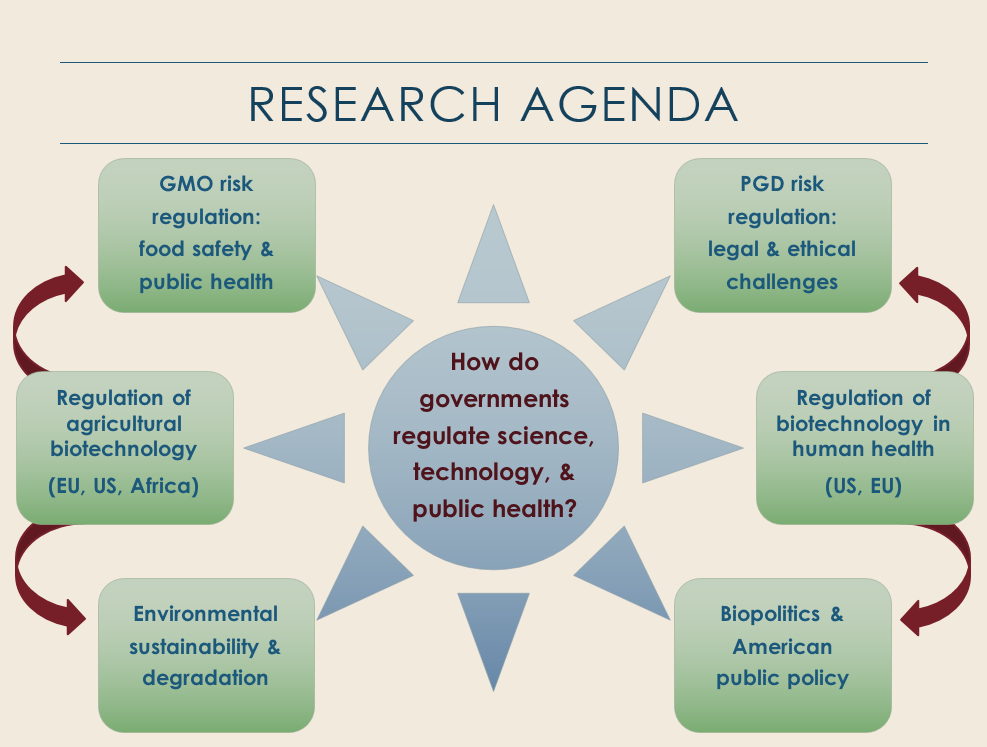 Visualization of Research Agenda
Visualization of Research AgendaWhile my dissertation was specific to the case of France within the context of the EU, I have also been pursuing comparative research on American and European agricultural biotechnology regulation and research on global governance in this policy domain. For example, in February 2015 I gave an invited talk at the University of Pittsburgh’s European Union Center of Excellence/European Studies Center on GMOs as an obstacle to the Transatlantic Trade and Investment Partnership (TTIP) negotiations. The talk followed a similar line of inquiry that I established in presentations at the NPSA’s Annual Meeting in November 2012 and the APSA Annual Meeting in August 2013. At those conferences, I presented research on the differences in the North American and European regulatory approaches, and how those differences play out in the case of institutional developments in agricultural biotechnology regulatory frameworks in Sub-Saharan African states. The next phase of my research in this area will include a more in-depth review of the regulatory developments in Kenya, Uganda, and Nigeria, and an analysis of the role of American biotechnology companies – particularly Monsanto – in shaping American foreign policy in these African states.
My expansion into using the United States as a case study for my comparative work has also led to several collaborations with Andrew Byers, a Historian at Duke University. Our edited volume, Biopolitics and Utopia, grew out of a panel we organized for the annual meeting of the Society for Utopian Studies (SUS) in 2013. The success of that collaboration has resulted in another panel for the upcoming 2015 SUS conference, as well as our current project: Biopolitics and American Public Policy. Building on the comparative methods of policy analysis that I used in my dissertation, this co-authored volume is a public policy text that applies the multiple streams model to seven issue areas in American politics: prison sentencing; domestic surveillance; marriage equality; prescription drug regulation; legalization of marijuana; childhood obesity; and gun control. Our book presents an interesting and timely review of contemporary policy areas, particularly in how we frame our case studies within the context of biopolitics, and it develops another branch of my research agenda.
 Behind the Brands by Joki Gauthier / Oxfam America
Behind the Brands by Joki Gauthier / Oxfam America